publications
selected publications
2025
- Detection of Epileptogenic Focal Cortical Dysplasia Using Graph Neural Networks: A MELD StudyMathilde Ripart, Hannah Spitzer, Logan Z J Williams, and 76 more authorsJAMA Neurol, Feb 2025
ImportanceA leading cause of surgically remediable, drug-resistant focal epilepsy is focal cortical dysplasia (FCD). FCD is challenging to visualize and often considered magnetic resonance imaging (MRI) negative. Existing automated methods for FCD detection are limited by high numbers of false-positive predictions, hampering their clinical utility.ObjectiveTo evaluate the efficacy and interpretability of graph neural networks in automatically detecting FCD lesions on MRI scans.Design, Setting, and ParticipantsIn this multicenter diagnostic study, retrospective MRI data were collated from 23 epilepsy centers worldwide between 2018 and 2022, as part of the Multicenter Epilepsy Lesion Detection (MELD) Project, and analyzed in 2023. Data from 20 centers were split equally into training and testing cohorts, with data from 3 centers withheld for site-independent testing. A graph neural network (MELD Graph) was trained to identify FCD on surface-based features. Network performance was compared with an existing algorithm. Feature analysis, saliencies, and confidence scores were used to interpret network predictions. In total, 34 surface-based MRI features and manual lesion masks were collated from participants, 703 patients with FCD–related epilepsy and 482 controls, and 57 participants were excluded during MRI quality control.Main Outcomes and MeasuresSensitivity, specificity, and positive predictive value (PPV) of automatically identified lesions.ResultsIn the test dataset, the MELD Graph had a sensitivity of 81.6% in histopathologically confirmed patients seizure-free 1 year after surgery and 63.7% in MRI–negative patients with FCD. The PPV of putative lesions from the 260 patients in the test dataset (125 female [48%] and 135 male [52%]; mean age, 18.0 [IQR, 11.0-29.0] years) was 67% (70% sensitivity; 60% specificity), compared with 39% (67% sensitivity; 54% specificity) using an existing baseline algorithm. In the independent test cohort (116 patients; 62 female [53%] and 54 male [47%]; mean age, 22.5 [IQR, 13.5-27.5] years), the PPV was 76% (72% sensitivity; 56% specificity), compared with 46% (77% sensitivity; 47% specificity) using the baseline algorithm. Interpretable reports characterize lesion location, size, confidence, and salient features.Conclusions and RelevanceIn this study, the MELD Graph represented a state-of-the-art, openly available, and interpretable tool for FCD detection on MRI scans with significant improvements in PPV. Its clinical implementation holds promise for early diagnosis and improved management of focal epilepsy, potentially leading to better patient outcomes.
2024
-
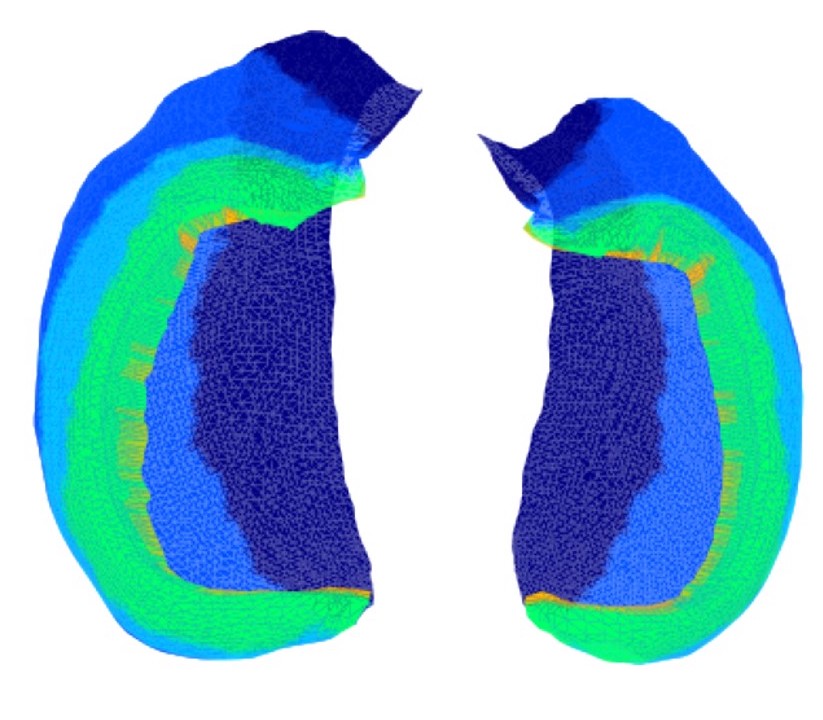 Automated and Interpretable Detection of Hippocampal Sclerosis in temporal lobe epilepsy: AID-HSMathilde Ripart, Jordan DeKraker, Maria H Eriksson, and 42 more authorsAnn. Neurol., Nov 2024
Automated and Interpretable Detection of Hippocampal Sclerosis in temporal lobe epilepsy: AID-HSMathilde Ripart, Jordan DeKraker, Maria H Eriksson, and 42 more authorsAnn. Neurol., Nov 2024OBJECTIVE: Hippocampal sclerosis (HS), the most common pathology associated with temporal lobe epilepsy (TLE), is not always visible on magnetic resonance imaging (MRI), causing surgical delays and reduced postsurgical seizure-freedom. We developed an open-source software to characterize and localize HS to aid the presurgical evaluation of children and adults with suspected TLE. METHODS: We included a multicenter cohort of 365 participants (154 HS; 90 disease controls; 121 healthy controls). HippUnfold was used to extract morphological surface-based features and volumes of the hippocampus from T1-weighted MRI scans. We characterized pathological hippocampi in patients by comparing them to normative growth charts and analyzing within-subject feature asymmetries. Feature asymmetry scores were used to train a logistic regression classifier to detect and lateralize HS. The classifier was validated on an independent multicenter cohort of 275 patients with HS and 161 healthy and disease controls. RESULTS: HS was characterized by decreased volume, thickness, and gyrification alongside increased mean and intrinsic curvature. The classifier detected 90.1% of unilateral HS patients and lateralized lesions in 97.4%. In patients with MRI-negative histopathologically-confirmed HS, the classifier detected 79.2% (19/24) and lateralized 91.7% (22/24). The model achieved similar performances on the independent cohort, demonstrating its ability to generalize to new data. Individual patient reports contextualize a patient’s hippocampal features in relation to normative growth trajectories, visualise feature asymmetries, and report classifier predictions. INTERPRETATION: Automated and Interpretable Detection of Hippocampal Sclerosis (AID-HS) is an open-source pipeline for detecting and lateralizing HS and outputting clinically-relevant reports. ANN NEUROL 2024.
-
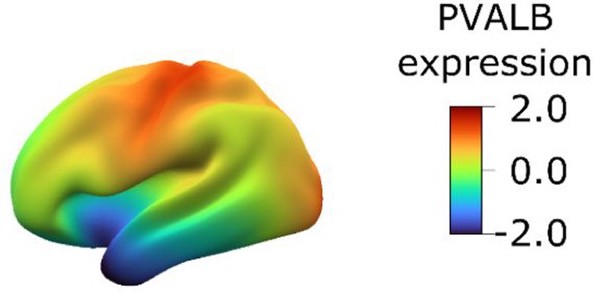 Transcriptional cartography integrates multiscale biology of the human cortexKonrad Wagstyl, Sophie Adler, Jakob Seidlitz, and 11 more authorsElife, Feb 2024
Transcriptional cartography integrates multiscale biology of the human cortexKonrad Wagstyl, Sophie Adler, Jakob Seidlitz, and 11 more authorsElife, Feb 2024The cerebral cortex underlies many of our unique strengths and vulnerabilities, but efforts to understand human cortical organization are challenged by reliance on incompatible measurement methods at different spatial scales. Macroscale features such as cortical folding and functional activation are accessed through spatially dense neuroimaging maps, whereas microscale cellular and molecular features are typically measured with sparse postmortem sampling. Here, we integrate these distinct windows on brain organization by building upon existing postmortem data to impute, validate, and analyze a library of spatially dense neuroimaging-like maps of human cortical gene expression. These maps allow spatially unbiased discovery of cortical zones with extreme transcriptional profiles or unusually rapid transcriptional change which index distinct microstructure and predict neuroimaging measures of cortical folding and functional activation. Modules of spatially coexpressed genes define a family of canonical expression maps that integrate diverse spatial scales and temporal epochs of human brain organization–ranging from protein–protein interactions to large-scale systems for cognitive processing. These module maps also parse neuropsychiatric risk genes into subsets which tag distinct cyto-laminar features and differentially predict the location of altered cortical anatomy and gene expression in patients. Taken together, the methods, resources, and findings described here advance our understanding of human cortical organization and offer flexible bridges to connect scientific fields operating at different spatial scales of human brain research.
2023
-
 Robust and Generalisable Segmentation of Subtle Epilepsy-Causing Lesions: A Graph Convolutional ApproachHannah Spitzer, Mathilde Ripart, Abdulah Fawaz, and 5 more authorsIn Medical Image Computing and Computer Assisted Intervention – MICCAI 2023 , Feb 2023
Robust and Generalisable Segmentation of Subtle Epilepsy-Causing Lesions: A Graph Convolutional ApproachHannah Spitzer, Mathilde Ripart, Abdulah Fawaz, and 5 more authorsIn Medical Image Computing and Computer Assisted Intervention – MICCAI 2023 , Feb 2023Focal cortical dysplasia (FCD) is a leading cause of drug-resistant focal epilepsy, which can be cured by surgery. These lesions are extremely subtle and often missed even by expert neuroradiologists. “Ground truth” manual lesion masks are therefore expensive, limited and have large inter-rater variability. Existing FCD detection methods are limited by high numbers of false positive predictions, primarily due to vertex- or patch-based approaches that lack whole-brain context. Here, we propose to approach the problem as semantic segmentation using graph convolutional networks (GCN), which allows our model to learn spatial relationships between brain regions. To address the specific challenges of FCD identification, our proposed model includes an auxiliary loss to predict distance from the lesion to reduce false positives and a weak supervision classification loss to facilitate learning from uncertain lesion masks. On a multi-centre dataset of 1015 participants with surface-based features and manual lesion masks from structural MRI data, the proposed GCN achieved an AUC of 0.74, a significant improvement against a previously used vertex-wise multi-layer perceptron (MLP) classifier (AUC 0.64). With sensitivity thresholded at 67%, the GCN had a specificity of 71% in comparison to 49% when using the MLP. This improvement in specificity is vital for clinical integration of lesion-detection tools into the radiological workflow, through increasing clinical confidence in the use of AI radiological adjuncts and reducing the number of areas requiring expert review.
-
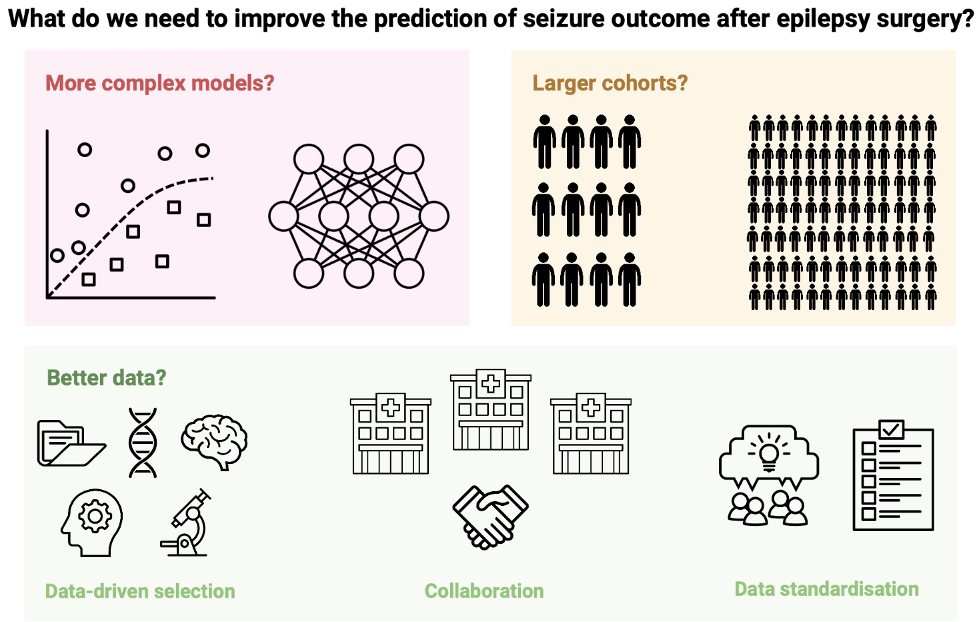 Predicting seizure outcome after epilepsy surgery: do we need more complex models, larger samples, or better data?Maria H Eriksson, Mathilde Ripart, Rory J Piper, and 16 more authorsEpilepsia, May 2023
Predicting seizure outcome after epilepsy surgery: do we need more complex models, larger samples, or better data?Maria H Eriksson, Mathilde Ripart, Rory J Piper, and 16 more authorsEpilepsia, May 2023OBJECTIVE: The accurate prediction of seizure freedom after epilepsy surgery remains challenging. We investigated if 1) training more complex models, 2) recruiting larger sample sizes, or 3) using data-driven selection of clinical predictors would improve our ability to predict post-operative seizure outcome using clinical features. We also conducted the first substantial external validation of a machine learning model trained to predict post-operative seizure outcome. METHODS: We performed a retrospective cohort study of 797 children who had undergone resective or disconnective epilepsy surgery at a tertiary center. We extracted patient information from medical records and trained three models - a logistic regression, a multilayer perceptron, and an XGBoost model - to predict one-year post-operative seizure outcome on our dataset. We evaluated the performance of a recently published XGBoost model on the same patients. We further investigated the impact of sample size on model performance, using learning curve analysis to estimate performance at samples up to N=2,000. Finally, we examined the impact of predictor selection on model performance. RESULTS: Our logistic regression achieved an accuracy of 72% (95% CI=68-75%,AUC=0.72), while our multilayer perceptron and XGBoost both achieved accuracies of 71% (95% CIMLP =67-74%,AUCMLP =0.70; 95% CIXGBoost own =68-75%,AUCXGBoost own =0.70). There was no significant difference in performance between our three models (all P>0.4) and they all performed better than the external XGBoost, which achieved an accuracy of 63% (95% CI=59-67%,AUC=0.62; PLR =0.005,PMLP =0.01,PXGBoost own =0.01) on our data. All models showed improved performance with increasing sample size, but limited improvements beyond our current sample. The best model performance was achieved with data-driven feature selection. SIGNIFICANCE: We show that neither the deployment of complex machine learning models nor the assembly of thousands of patients alone is likely to generate significant improvements in our ability to predict post-operative seizure freedom. We instead propose that improved feature selection alongside collaboration, data standardization, and model sharing is required to advance the field.
2022
- Interpretable surface-based detection of focal cortical dysplasias: a Multi-centre Epilepsy Lesion Detection studyHannah Spitzer, Mathilde Ripart, Kirstie Whitaker, and 74 more authorsBrain, Nov 2022
One outstanding challenge for machine learning in diagnostic biomedical imaging is algorithm interpretability. A key application is the identification of subtle epileptogenic focal cortical dysplasias (FCDs) from structural MRI. FCDs are difficult to visualize on structural MRI but are often amenable to surgical resection. We aimed to develop an open-source, interpretable, surface-based machine-learning algorithm to automatically identify FCDs on heterogeneous structural MRI data from epilepsy surgery centres worldwide. The Multi-centre Epilepsy Lesion Detection (MELD) Project collated and harmonized a retrospective MRI cohort of 1015 participants, 618 patients with focal FCD-related epilepsy and 397 controls, from 22 epilepsy centres worldwide. We created a neural network for FCD detection based on 33 surface-based features. The network was trained and cross-validated on 50% of the total cohort and tested on the remaining 50% as well as on 2 independent test sites. Multidimensional feature analysis and integrated gradient saliencies were used to interrogate network performance. Our pipeline outputs individual patient reports, which identify the location of predicted lesions, alongside their imaging features and relative saliency to the classifier. On a restricted ’gold-standard’ subcohort of seizure-free patients with FCD type IIB who had T1 and fluid-attenuated inversion recovery MRI data, the MELD FCD surface-based algorithm had a sensitivity of 85%. Across the entire withheld test cohort the sensitivity was 59% and specificity was 54%. After including a border zone around lesions, to account for uncertainty around the borders of manually delineated lesion masks, the sensitivity was 67%. This multicentre, multinational study with open access protocols and code has developed a robust and interpretable machine-learning algorithm for automated detection of focal cortical dysplasias, giving physicians greater confidence in the identification of subtle MRI lesions in individuals with epilepsy.
2021
-
 Atlas of lesion locations and postsurgical seizure freedom in focal cortical dysplasia: A MELD studyKonrad Wagstyl, Kirstie Whitaker, Armin Raznahan, and 64 more authorsEpilepsia, Nov 2021
Atlas of lesion locations and postsurgical seizure freedom in focal cortical dysplasia: A MELD studyKonrad Wagstyl, Kirstie Whitaker, Armin Raznahan, and 64 more authorsEpilepsia, Nov 2021OBJECTIVE: Drug-resistant focal epilepsy is often caused by focal cortical dysplasias (FCDs). The distribution of these lesions across the cerebral cortex and the impact of lesion location on clinical presentation and surgical outcome are largely unknown. We created a neuroimaging cohort of patients with individually mapped FCDs to determine factors associated with lesion location and predictors of postsurgical outcome. METHODS: The MELD (Multi-centre Epilepsy Lesion Detection) project collated a retrospective cohort of 580 patients with epilepsy attributed to FCD from 20 epilepsy centers worldwide. Magnetic resonance imaging-based maps of individual FCDs with accompanying demographic, clinical, and surgical information were collected. We mapped the distribution of FCDs, examined for associations between clinical factors and lesion location, and developed a predictive model of postsurgical seizure freedom. RESULTS: FCDs were nonuniformly distributed, concentrating in the superior frontal sulcus, frontal pole, and temporal pole. Epilepsy onset was typically before the age of 10 years. Earlier epilepsy onset was associated with lesions in primary sensory areas, whereas later epilepsy onset was associated with lesions in association cortices. Lesions in temporal and occipital lobes tended to be larger than frontal lobe lesions. Seizure freedom rates varied with FCD location, from around 30% in visual, motor, and premotor areas to 75% in superior temporal and frontal gyri. The predictive model of postsurgical seizure freedom had a positive predictive value of 70% and negative predictive value of 61%. SIGNIFICANCE: FCD location is an important determinant of its size, the age at epilepsy onset, and the likelihood of seizure freedom postsurgery. Our atlas of lesion locations can be used to guide the radiological search for subtle lesions in individual patients. Our atlas of regional seizure freedom rates and associated predictive model can be used to estimate individual likelihoods of postsurgical seizure freedom. Data-driven atlases and predictive models are essential for evidence-based, precision medicine and risk counseling in epilepsy.
2020
- BigBrain 3D atlas of cortical layers: Cortical and laminar thickness gradients diverge in sensory and motor corticesKonrad Wagstyl, Stéphanie Larocque, Guillem Cucurull, and 14 more authorsPLoS Biol., Apr 2020
Histological atlases of the cerebral cortex, such as those made famous by Brodmann and von Economo, are invaluable for understanding human brain microstructure and its relationship with functional organization in the brain. However, these existing atlases are limited to small numbers of manually annotated samples from a single cerebral hemisphere, measured from 2D histological sections. We present the first whole-brain quantitative 3D laminar atlas of the human cerebral cortex. It was derived from a 3D histological atlas of the human brain at 20-micrometer isotropic resolution (BigBrain), using a convolutional neural network to segment, automatically, the cortical layers in both hemispheres. Our approach overcomes many of the historical challenges with measurement of histological thickness in 2D, and the resultant laminar atlas provides an unprecedented level of precision and detail. We utilized this BigBrain cortical atlas to test whether previously reported thickness gradients, as measured by MRI in sensory and motor processing cortices, were present in a histological atlas of cortical thickness and which cortical layers were contributing to these gradients. Cortical thickness increased across sensory processing hierarchies, primarily driven by layers III, V, and VI. In contrast, motor-frontal cortices showed the opposite pattern, with decreases in total and pyramidal layer thickness from motor to frontal association cortices. These findings illustrate how this laminar atlas will provide a link between single-neuron morphology, mesoscale cortical layering, macroscopic cortical thickness, and, ultimately, functional neuroanatomy.
2017
- Novel surface features for automated detection of focal cortical dysplasias in paediatric epilepsySophie Adler, Konrad Wagstyl, Roxana Gunny, and 5 more authorsNeuroimage Clin, Apr 2017
Focal cortical dysplasia is a congenital abnormality of cortical development and the leading cause of surgically remediable drug-resistant epilepsy in children. Post-surgical outcome is improved by presurgical lesion detection on structural MRI. Automated computational techniques have improved detection of focal cortical dysplasias in adults but have not yet been effective when applied to developing brains. There is therefore a need to develop reliable and sensitive methods to address the particular challenges of a paediatric cohort. We developed a classifier using surface-based features to identify focal abnormalities of cortical development in a paediatric cohort. In addition to established measures, such as cortical thickness, grey-white matter blurring, FLAIR signal intensity, sulcal depth and curvature, our novel features included complementary metrics of surface morphology such as local cortical deformation as well as post-processing methods such as the “doughnut” method - which quantifies local variability in cortical morphometry/MRI signal intensity, and per-vertex interhemispheric asymmetry. A neural network classifier was trained using data from 22 patients with focal epilepsy (mean age = 12.1 \pm 3.9, 9 females), after intra- and inter-subject normalisation using a population of 28 healthy controls (mean age = 14.6 \pm 3.1, 11 females). Leave-one-out cross-validation was used to quantify classifier sensitivity using established features and the combination of established and novel features. Focal cortical dysplasias in our paediatric cohort were correctly identified with a higher sensitivity (73%) when novel features, based on our approach for detecting local cortical changes, were included, when compared to the sensitivity using only established features (59%). These methods may be applicable to aiding identification of subtle lesions in medication-resistant paediatric epilepsy as well as to the structural analysis of both healthy and abnormal cortical development.